what two proteins regulate the attachment of the myosin head to the actin binding site
Actin filaments, ordinarily in association with myosin, are responsible for many types of cell movements. Myosin is the prototype of a molecular motor—a protein that converts chemical energy in the form of ATP to mechanical energy, thus generating forcefulness and movement. The most striking variety of such movement is muscle contraction, which has provided the model for understanding actin-myosin interactions and the motor activity of myosin molecules. However, interactions of actin and myosin are responsible non but for musculus contraction but also for a variety of movements of nonmuscle cells, including jail cell sectionalisation, and so these interactions play a primal role in prison cell biology. Moreover, the actin cytoskeleton is responsible for the crawling movements of cells across a surface, which announced to exist driven straight past actin polymerization also equally actin-myosin interactions.
Muscle Wrinkle
Musculus cells are highly specialized for a unmarried job, contraction, and it is this specialization in structure and office that has made muscle the prototype for studying movement at the cellular and molecular levels. There are three singled-out types of muscle cells in vertebrates: skeletal musculus, which is responsible for all voluntary movements; cardiac muscle, which pumps blood from the heart; and smooth muscle, which is responsible for involuntary movements of organs such as the stomach, intestine, uterus, and blood vessels. In both skeletal and cardiac musculus, the contractile elements of the cytoskeleton are nowadays in highly organized arrays that give rising to characteristic patterns of cantankerous-striations. It is the characterization of these structures in skeletal musculus that has led to our current agreement of muscle contraction, and other actin-based cell movements, at the molecular level.
Skeletal muscles are bundles of muscle fibers, which are single large cells (approximately 50 μm in diameter and up to several centimeters in length) formed past the fusion of many individual cells during evolution (Figure 11.18). Most of the cytoplasm consists of myofibrils, which are cylindrical bundles of two types of filaments: thick filaments of myosin (about 15 nm in diameter) and sparse filaments of actin (about 7 nm in diameter). Each myofibril is organized every bit a chain of contractile units chosen sarcomeres, which are responsible for the striated appearance of skeletal and cardiac muscle.

Figure xi.18
Structure of musculus cells. Muscles are equanimous of bundles of single large cells (called muscle fibers) that form by cell fusion and incorporate multiple nuclei. Each musculus fiber contains many myofibrils, which are bundles of actin and myosin filaments organized (more than...)
The sarcomeres (which are approximately ii.3 μm long) consist of several distinct regions, discernible past electron microscopy, which provided disquisitional insights into the mechanism of muscle contraction (Figure 11.nineteen). The ends of each sarcomere are defined past the Z disc. Within each sarcomere, night bands (called A bands because they are anisotropic when viewed with polarized light) alternating with light bands (called I bands for isotropic). These bands represent to the presence or absence of myosin filaments. The I bands incorporate only thin (actin) filaments, whereas the A bands contain thick (myosin) filaments. The myosin and actin filaments overlap in peripheral regions of the A band, whereas a eye region (called the H zone) contains but myosin. The actin filaments are attached at their plus ends to the Z disc, which includes the crosslinking poly peptide α-actinin. The myosin filaments are anchored at the M line in the center of the sarcomere.

Figure eleven.19
Structure of the sarcomere. (A) Electron micrograph of a sarcomere. (B) Diagram showing the organization of actin (thin) and myosin (thick) filaments in the indicated regions. (A, Frank A. Pepe/Biological Photo Service.)
2 additional proteins (titin and nebulin) also contribute to sarcomere structure and stability (Figure 11.twenty). Titin is an extremely large protein (3000 kd), and single titin molecules extend from the M line to the Z disc. These long molecules of titin are idea to act like springs that keep the myosin filaments centered in the sarcomere and maintain the resting tension that allows a muscle to snap back if overextended. Nebulin filaments are associated with actin and are idea to regulate the associates of actin filaments past interim as rulers that make up one's mind their length.

Figure 11.xx
Titin and nebulin. Molecules of titin extend from the Z disc to the M line and deed as springs to continue myosin filaments centered in the sarcomere. Molecules of nebulin extend from the Z disc and are thought to determine the length of associated actin filaments. (more than...)
The basis for agreement muscle contraction is the sliding filament model, outset proposed in 1954 both by Andrew Huxley and Ralph Niedergerke and by Hugh Huxley and Jean Hanson (Figure 11.21). During muscle contraction, each sarcomere shortens, bringing the Z discs closer together. There is no change in the width of the A band, but both the I bands and the H zone almost completely disappear. These changes are explained by the actin and myosin filaments sliding past 1 another, so that the actin filaments move into the A ring and H zone. Muscle contraction thus results from an interaction between the actin and myosin filaments that generates their movement relative to one another. The molecular ground for this interaction is the binding of myosin to actin filaments, allowing myosin to role every bit a motor that drives filament sliding.
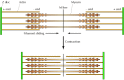
Figure eleven.21
Sliding-filament model of muscle contraction. The actin filaments slide past the myosin filaments toward the eye of the sarcomere. The result is shortening of the sarcomere without whatsoever modify in filament length.
The type of myosin nowadays in muscle (myosin II) is a very large poly peptide (almost 500 kd) consisting of two identical heavy chains (about 200 kd each) and two pairs of light chains (about 20 kd each) (Figure eleven.22). Each heavy chain consists of a globular head region and a long α-helical tail. The α-helical tails of two heavy chains twist around each other in a coiled-curlicue structure to class a dimer, and two light chains associate with the cervix of each head region to form the complete myosin II molecule.
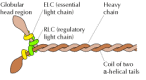
Figure 11.22
Myosin II. The myosin II molecule consists of two heavy chains and two pairs of light bondage (called the essential and regulatory light chains). The heavy chains have globular head regions and long α-helical tails, which coil around each other (more...)
The thick filaments of muscle consist of several hundred myosin molecules, associated in a parallel staggered assortment by interactions betwixt their tails (Figure 11.23). The globular heads of myosin bind actin, forming cross-bridges between the thick and thin filaments. It is important to note that the orientation of myosin molecules in the thick filaments reverses at the Grand line of the sarcomere. The polarity of actin filaments (which are attached to Z discs at their plus ends) similarly reverses at the Yard line, and then the relative orientation of myosin and actin filaments is the same on both halves of the sarcomere. As discussed later, the motor activity of myosin moves its head groups forth the actin filament in the direction of the plus end. This movement slides the actin filaments from both sides of the sarcomere toward the G line, shortening the sarcomere and resulting in muscle wrinkle.

Figure 11.23
Organization of myosin thick filaments. Thick filaments are formed by the clan of several hundred myosin Two molecules in a staggered array. The globular heads of myosin demark actin, forming cross-bridges between the myosin and actin filaments. The (more...)
In addition to binding actin, the myosin heads bind and hydrolyze ATP, which provides the energy to drive filament sliding. This translation of chemic energy to movement is mediated by changes in the shape of myosin resulting from ATP binding. The generally accustomed model (the swinging-cross-bridge model) is that ATP hydrolysis drives repeated cycles of interaction betwixt myosin heads and actin. During each cycle, conformational changes in myosin result in the move of myosin heads along actin filaments.
Although the molecular mechanisms are still not fully understood, a plausible working model for myosin office has been derived both from in vitro studies of myosin movement forth actin filaments (a system developed by James Spudich and Michael Sheetz) and from determination of the iii-dimensional structure of myosin by Ivan Rayment and his colleagues (Figure xi.24). The cycle starts with myosin (in the absence of ATP) tightly bound to actin. ATP binding dissociates the myosin-actin complex and the hydrolysis of ATP and then induces a conformational change in myosin. This change affects the neck region of myosin that binds the light chains (see Figure 11.22), which acts as a lever arm to displace the myosin head past about 5 nm. The products of hydrolysis (ADP and P i ) remain bound to the myosin caput, which is said to exist in the "cocked" position. The myosin caput and so rebinds at a new position on the actin filament, resulting in the release of ADP and P i and triggering the "power stroke," in which the myosin caput returns to its initial conformation, thereby sliding the actin filament toward the M line of the sarcomere.
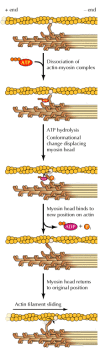
Figure xi.24
Model for myosin action. The binding of ATP dissociates myosin from actin. ATP hydrolysis and then induces a conformational modify that displaces the myosin head group. This is followed past binding of the myosin head to a new position on the actin filament (more than...)
The contraction of skeletal muscle is triggered by nervus impulses, which stimulate the release of Ca2+ from the sarcoplasmic reticulum—a specialized network of internal membranes, similar to the endoplasmic reticulum, that stores high concentrations of Ca2+ ions. The release of Ca2+ from the sarcoplasmic reticulum increases the concentration of Catwo+ in the cytosol from approximately 10-7 to x-five M. The increased Caii+ concentration signals musculus contraction via the action of ii accompaniment proteins bound to the actin filaments: tropomyosin and troponin (Figure xi.25). Tropomyosin is a fibrous poly peptide that binds lengthwise along the groove of actin filaments. In striated muscle, each tropomyosin molecule is leap to troponin, which is a circuitous of three polypeptides: troponin C (Ca2+-binding), troponin I (inhibitory), and troponin T (tropomyosin-binding). When the concentration of Caii+ is depression, the circuitous of the troponins with tropomyosin blocks the interaction of actin and myosin, then the muscle does non contract. At loftier concentrations, Ca2+ binding to troponin C shifts the position of the complex, relieving this inhibition and allowing contraction to proceed.

Figure 11.25
Clan of tropomyosin and troponins with actin filaments. (A) Tropomyosin binds lengthwise forth actin filaments and, in striated muscle, is associated with a complex of three troponins: troponin I (TnI), troponin C (TnC), and troponin T (TnT). In (more...)
Contractile Assemblies of Actin and Myosin in Nonmuscle Cells
Contractile assemblies of actin and myosin, resembling small-scale versions of muscle fibers, are present also in nonmuscle cells. Every bit in muscle, the actin filaments in these contractile assemblies are interdigitated with bipolar filaments of myosin II, consisting of fifteen to twenty myosin II molecules, which produce contraction by sliding the actin filaments relative to ane another (Figure eleven.26). The actin filaments in contractile bundles in nonmuscle cells are besides associated with tropomyosin, which facilitates their interaction with myosin Two, probably past competing with filamin for binding sites on actin.

Figure 11.26
Contractile assemblies in nonmuscle cells. Bipolar filaments of myosin II produce contraction past sliding actin filaments in opposite directions.
Two examples of contractile assemblies in nonmuscle cells, stress fibers and adhesion belts, were discussed earlier with respect to attachment of the actin cytoskeleton to regions of cell-substrate and jail cell-cell contacts (encounter Figures eleven.13 and 11.14). The wrinkle of stress fibers produces tension across the cell, allowing the cell to pull on a substrate (e.one thousand., the extracellular matrix) to which it is anchored. The contraction of adhesion belts alters the shape of epithelial cell sheets: a process that is particularly important during embryonic development, when sheets of epithelial cells fold into structures such as tubes.
The most dramatic case of actin-myosin wrinkle in nonmuscle cells, however, is provided by cytokinesis—the division of a cell into two following mitosis (Figure 11.27). Toward the end of mitosis in animal cells, a contractile band consisting of actin filaments and myosin II assembles just underneath the plasma membrane. Its contraction pulls the plasma membrane progressively inward, constricting the center of the cell and pinching information technology in 2. Interestingly, the thickness of the contractile ring remains abiding as it contracts, implying that actin filaments disassemble equally contraction proceeds. The ring and so disperses completely post-obit cell partition.
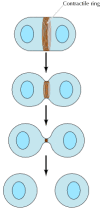
Figure 11.27
Cytokinesis. Following completion of mitosis (nuclear sectionalisation), a contractile band consisting of actin filaments and myosin II divides the cell in two.
The regulation of actin-myosin contraction in striated musculus, discussed earlier, is mediated by the binding of Ca2+ to troponin. In nonmuscle cells and in smooth muscle, yet, contraction is regulated primarily by phosphorylation of one of the myosin lite chains, called the regulatory light chain (Figure 11.28). Phosphorylation of the regulatory light concatenation in these cells has at least two effects: It promotes the assembly of myosin into filaments, and information technology increases myosin catalytic activity, enabling wrinkle to proceed. The enzyme that catalyzes this phosphorylation, called myosin lite-concatenation kinase, is itself regulated by association with the Ca2+-binding protein calmodulin. Increases in cytosolic Ca2+ promote the binding of calmodulin to the kinase, resulting in phosphorylation of the myosin regulatory light chain. Increases in cytosolic Caii+ are thus responsible, admitting indirectly, for activating myosin in shine muscle and nonmuscle cells, as well as in striated muscle.

Figure 11.28
Regulation of myosin by phosphorylation. Ca2+ binds to calmodulin, which in plough binds to myosin light-concatenation kinase (MLCK). The active calmodulin-MLCK complex then phosphorylates the myosin 2 regulatory low-cal chain, converting myosin from an inactive (more...)
Unconventional Myosins
In add-on to myosin Two ("conventional" two-headed myosin), several other types of myosin are found in nonmuscle cells. In contrast to myosin II, these "unconventional" myosins do not grade filaments and therefore are non involved in contraction. They may, however, be involved in a variety of other kinds of cell movements, such as the transport of membrane vesicles and organelles forth actin filaments, phagocytosis, and extension of pseudopods in amoebae (meet Figure xi.17).
The best-studied of these unconventional myosins are members of the myosin I family (Figure 11.29). The myosin I proteins contain a globular head group that acts every bit a molecular motor, like that of myosin II. Withal, members of the myosin I family unit are much smaller molecules (virtually 110 kd in mammalian cells) that lack the long tail of myosin II and do non form dimers. Their tails tin instead bind to other structures, such as membrane vesicles or organelles. The motion of myosin I along an actin filament tin so transport its attached cargo. Ane office of myosin I, discussed earlier, is to form the lateral arms that link actin bundles to the plasma membrane of abdominal microvilli (see Effigy 11.16). In these structures, the motor activeness of myosin I may motion the plasma membrane forth the actin bundles, toward the tip of the microvillus. Boosted functions of myosin I may be in the transport of vesicles and organelles along actin filaments and in motility of the plasma membrane during phagocytosis and pseudopod extension.
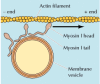
Figure 11.29
Myosin I. Myosin I contains a head group similar to myosin II, but it has a comparatively short tail and does not grade dimers or filaments. Although information technology cannot induce contraction, myosin I can move along actin filaments (toward the plus stop), conveying (more than...)
In improver to myosins I and II, at least 12 other classes of unconventional myosins (III through Fourteen) have been identified. Some of these unconventional myosins are ii-headed like myosin II, whereas others are one-headed like myosin I. The functions of almost of these unconventional myosins remain to be determined, but some have been conspicuously shown to play important roles in organelle move (myosins V and Half dozen) and in sensory functions such equally vision (myosin III) and hearing (myosins Vi and VII).
Cell Crawling
The crawling movements of cells across a surface represent a basic grade of jail cell locomotion, employed by a wide multifariousness of dissimilar kinds of cells. Examples include the movements of amoebas, the migration of embryonic cells during development, the invasion of tissues by white blood cells to fight infection, the migration of cells involved in wound healing, and the spread of cancer cells during the metastasis of malignant tumors. Similar types of movement are also responsible for phagocytosis and for the extension of nerve cell processes during development of the nervous organization. All of these movements are based on the dynamic properties of the actin cytoskeleton, although the detailed mechanisms involved remain to be fully understood.
Jail cell crawling involves a coordinated bike of movements, which can exist viewed in three stages. First, protrusions such as pseudopodia, lamellipodia, or microspikes (see Effigy 11.17) must be extended from the leading edge of the cell (Figure 11.xxx). Second, these extensions must attach to the substratum across which the cell is migrating. Finally, the abaft edge of the cell must dissociate from the substratum and retract into the cell body.
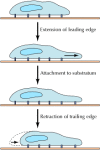
Figure 11.30
Cell itch. The crawling movements of cells across a surface can be viewed every bit three stages of coordinated movements: (1) extension of the leading edge, (ii) attachment of the leading edge to the substratum, and (iii) retraction of the rear of the cell (more...)
A diversity of experiments signal that extension of the leading edge involves the polymerization and crosslinking of actin filaments. For example, inhibition of actin polymerization (e.thousand., by handling with cytochalasin) blocks the germination of cell surface protrusions. The regulated turnover of actin filaments, as illustrated in Figure 11.5, leads to the extension of processes such as filopodia and lamellipodia at the leading border of the prison cell, and both cofilin and Arp2/three proteins announced to be involved in this process. Unconventional myosins may also participate in the extension of processes at the leading edge: Myosin I is required for pseudopod extension in the amoeba Dictyostelium and Myosin V for extension of filopodia in neurons.
Following their extension, protrusions from the leading border must attach to the substratum in order to function in cell locomotion. For ho-hum-moving cells, such as fibroblasts, attachment involves the formation of focal adhesions (encounter Effigy 11.13). Cells moving more rapidly, such as amoebas or white blood cells, form more than diffuse contacts with the substratum, the molecular limerick of which is non known.
The third stage of cell itch, retraction of the trailing border, is the least understood. The attachments of the trailing edge to the substratum are broken, and the rear of the prison cell recoils into the jail cell body. The process appears to require the development of tension between the front and rear of the cell, generating contractile force that eventually pulls the rear of the prison cell forward. This attribute of cell locomotion is impaired in mutants of Dictyostelium defective myosin II, consistent with a role for myosin Ii in contracting the actin cortex and generating the force required for retraction of the abaft edge.
Source: https://www.ncbi.nlm.nih.gov/books/NBK9961/
0 Response to "what two proteins regulate the attachment of the myosin head to the actin binding site"
ارسال یک نظر